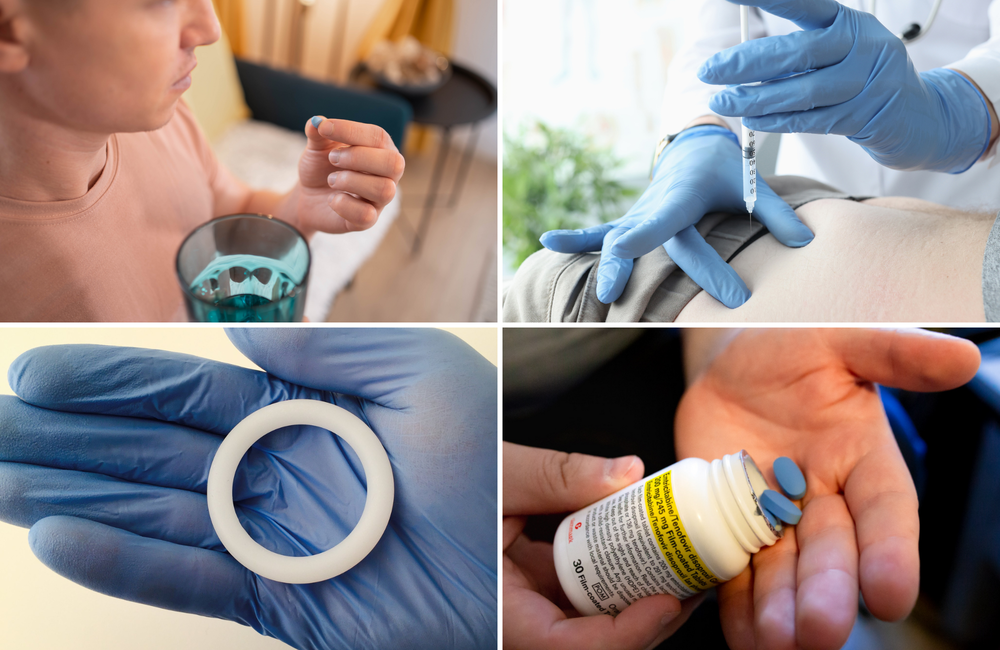
For decades, the global fight against HIV has remained persistent and filled with scientific breakthroughs amidst various challenges. Among these breakthroughs, the rollout of Pre-Exposure Prophylaxis (PrEP) has been a major milestone in the prevention of HIV, giving hope to millions of people at risk of contracting the virus. PrEP was conceptualized in the early 2000s as a means to prevent HIV transmission among high-risk populations. It involves the use of antiretroviral medications by persons without HIV to prevent the virus from establishing an infection in the body. In 2012, the U.S. Food and Drug Administration (FDA) approved the first PrEP regimen, Truvada (tenofovir disoproxil fumarate/emtricitabine), which marked a key moment in HIV prevention science.
The approval of Truvada came as a result of its remarkable efficacy in clinical trials. For instance, the iPrEx study in 2010 showed that daily Truvada reduced HIV risk by 44% overall and by over 90% among participants who adhered closely to the regimen. Similar studies, like the Partners PrEP study among heterosexual couples in Africa, reported risk reductions of up to 75%, showing the efficacy of the drug as a means to curb the HIV epidemic. An alternative to Truvada is Descovy (tenofovir alafenamide and emtricitabine), which was approved by the FDA in 2019 for use as oral PrEP, especially for sexually active men and transgender women at risk of HIV.
Expanding the PrEP Toolkit: Long-Acting and Alternative Options
Despite the availability of daily oral PrEP, some challenges, especially adherence and accompanying stigma, have limited its ease of use. This warranted further research into developing alternative PrEP options that will be more convenient and easier to adhere to. These relatively new options include long-acting injectable Cabotegravir, the Dapivirine vaginal ring, and the more recent injectable, Lenacapavir.
Long-Acting Cabotegravir (CAB-LA)
Cabotegravir, a long-acting injectable PrEP approved by the FDA in 2021, has been useful in HIV prevention. It is administered every two months, and is a great alternative for individuals who struggle with adhering to daily PrEP pills. Clinical trials like HPTN 083 and HPTN 084 demonstrated its superior efficacy compared to Truvada, reducing HIV acquisition by 66% and 89%, respectively. The introduction of injectable PrEP is important because it significantly reduces the adherence problem of using daily oral regimen, and also reduces stigma, as injections are discreet and eliminate the need for carrying pills
Dapivirine Vaginal Ring (DVR)
A more discreet PrEP option – the dapivirine vaginal ring, has been approved for use in several African countries. When inserted into the vagina, the silicone ring slowly releases the antiretroviral drug dapivirine, providing localized protection against HIV infection within a month period. According to reports from two separate clinical trials, the Ring Study demonstrated an HIV reduction of 35% among women using DVR, while the ASPIRE study recorded a 27% reduction in risk. Further open-label extension of the trials showed greater risk reduction by over 50% among women who consistently used the ring, implying a commendable level of efficacy.
The acceptance of the ring has been tested in the CATALYST study, involving over 3,900 women across five African countries. As presented by Dr. Elizabeth Irungu, Regional Technical Advisor for Implementation Science at Jhpiego, the study results showed that 30% of the women chose the ring for its convenience (especially pregnant and breastfeeding women), while 66% preferred to stick with oral PrEP. While most chose oral PrEP, the growing preference for the ring demonstrates its potential to offer women increased freedom over their HIV prevention options.
Furthermore, a three-month version of the ring has been developed to offer greater convenience and cost-effectiveness. The results from a phase 1 clinical trial showed that 72% of women preferred the 3-month ring over the one-month version, although some expressed concern about the hygiene and safety of the 3-month ring despite its increased convenience. As stated by Jeremy Nuttall, Senior Director of Preclinical Sciences for the Population Council, “It is quite clear that research in the field of HIV prevention is increasingly focused on expanding choice for women, and on long-acting prevention tools. In fact, the dapivirine vaginal ring was the first long-acting PrEP tool to receive a positive regulatory assessment.” He further stated that the three-month ring offered more benefits as it reduced the number of rings and packaging materials required per year (four rings a year instead of 12), and its estimated cost is about $16 US dollars, representing a 60% reduction in yearly cost compared to the one-month ring.
Twice-Yearly Lenacapavir
Lenacapavir, a subcutaneous injectable currently under regulatory review, has demonstrated exceptional promise in trials like PURPOSE 1 and 2. This drug, administered every six months, achieved HIV prevention rates of 96% to 100% in diverse populations, including cisgender women and men who have sex with men. Its long-acting nature makes it an important PrEP alternative in resource-limited settings where frequent healthcare visits are challenging. To make lenacapavir more accessible, the company Gilead Sciences has announced agreements with specific pharmaceutical companies for the production and distribution of the drug across low and middle-income countries to enable them obtain affordable versions of the drug once it is approved.

The Impact of PrEP and Future Directions
PrEP has already made a significant impact in reducing HIV incidence in many high-income countries. For instance, in the United States, PrEP has contributed to a 73% decline in new HIV diagnoses among MSM in major cities like San Francisco and New York. Similar trends have been observed in several other developed nations, and the consistent use of PrEP has been instrumental to the global decline in new HIV infections by 39% since 2010, and a 60% decline since the peak in 1995. In sub-Saharan Africa, where two-thirds of new HIV infections occur, PrEP rollout has been slower but is gaining momentum. Programs like the DREAMS initiative, which targets adolescent girls and young women, have helped integrate PrEP into broader HIV prevention strategies at a progressive rate.
The future of PrEP lies in innovation and progress is dependent on uptake, especially among key populations. More research is being made around ultra-long-acting options, such as subdermal implants that deliver antiretrovirals over a year or more. Experimental vaccines and broadly neutralizing antibodies (bNAbs) are also being studied as complementary strategies to enhance PrEP efficacy.
More importantly, while sustaining research and development in the field of HIV prevention, it is necessary to improve public access and use by integrating new PrEP products into healthcare systems – including sexual and reproductive health services. The ultimate goal is to create a world where PrEP is accessible, affordable, and acceptable to all who need it.