A Donor Bill of Rights

Source: International Disability Alliance. https://bit.ly/3t2C1mZ
The COVID-19 outbreak remains one of the biggest public health threats ever experienced by mankind since the history of infectious diseases. Currently, over 216 million cases and 4.5 million deaths have occurred worldwide, causing a great deal of panic, decrease in healthcare access, and economic distress. No widely approved treatment currently exists for COVID-19; however, a vital tool currently being used to fight off the disease is vaccination. Vaccines are substances containing the weakened version of an infectious pathogen, usually given to healthy individuals to elicit a protective immune response that prevents them from getting infected even in subsequent encounter with that same pathogen.
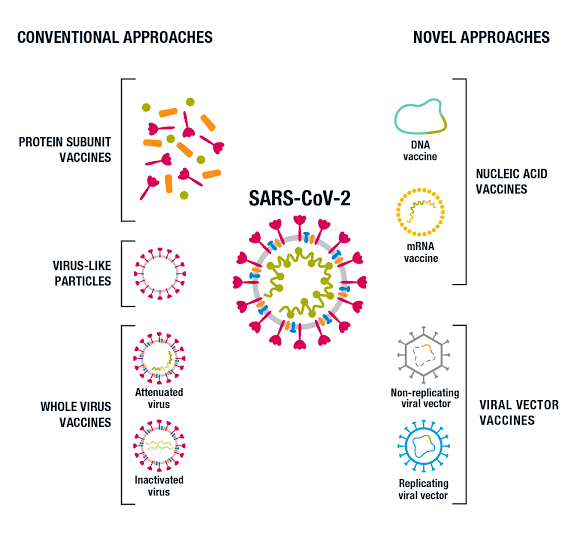
The term “COVID-19 Vaccine” actually refers to an array of approved vaccines produced by successful, top pharmaceutical companies that have shown high efficacy against COVID-19 and are fit to be used as an effective preventive measure. Since the onset of the pandemic, a lot of vaccine development firms and laboratories have been in the race to produce a suitable COVID-19 vaccine. Most of the vaccines undergoing production include vaccine types such as Inactivated virus vaccines, Protein subunit vaccines, Viral vector vaccines and DNA/RNA vaccines.
Initially, over 165 vaccine candidates were under research and development, but to date, only about 22 of them have received emergency use authorization (EUA) by national regulatory authorities. Out of these, only about 7 of them have been approved for full or emergency use by stringent regulatory authorities recognized by the World Health Organization (WHO). Such vaccines include Pfizer BioNTech, Oxford-AstraZeneca, Moderna, Sinopharm-BBIBP, Johnson and Johnson, and Sinovac (Covaxin). So far, the most widely used of these are the Pfizer BioNTech, Oxford-AstraZeneca and Moderna vaccines, which are currently authorized for use in the United States. The Food and Drug Administration (FDA) of the United States recently granted full authorization for the Pfizer BioNTech on August 23, 2021. This means that the vaccine has shown to be satisfactorily effective and safe for administration to all population types. The FDA only gives full authorization when it has been able to gather enough data on the efficacy of the vaccine and has also reviewed and approved the whole vaccine manufacturing process and facilities used. Note that emergency use authorization is only granted for certain vaccines when there is an urgent need to protect people against the spread of a highly infectious disease, and preliminary data from clinical trials have shown the vaccine to be effective, with very low adverse effects. This is done early in the vaccine review process, but over time, full authorization is granted when the FDA has amassed much more scientific evidence supporting the safety and efficacy of the vaccine for consistent use.
Since December 2020 when the first COVID-19 vaccine was approved for emergency use, over 5.2 billion vaccine doses have been administered worldwide, meaning that about 33.2% of the world’s population has received at least one dose of the COVID-19 vaccine so far. On average, about 38 million vaccine doses are administered each day, mostly to individuals from 16 years of age and above.
Source: NPS Medicinewise. https://bit.ly/3kKjecb
Generally, vaccines work by training the immune system to recognize and clear out disease-causing microbial pathogens. Vaccines contain either the weakened or killed versions of a virus, or a small part of the virus such as its spike proteins or nucleic acid. Basically, the vaccine mimics the actual viral pathogen, and when the vaccine is administered into a person, the immune system senses the virus or its proteins as harmful foreign bodies and elicits an immune response by producing antibodies against them. Antibodies are proteins produced by specific cells of the immune system that work by binding to pathogens and neutralizing them through a cascade of complex mechanisms. The vaccine acts to ‘sensitize’ the immune system against that pathogen in such a way that whenever the actual pathogen infects a person, the immune system immediately recognizes it without delay and neutralizes it before it can cause any harm. To put this in a simple way, you can think of a vaccine as a false alarm that alerts your immune system about a particular microbe, then when that microbe eventually enters your body, your immune system (which is already alert) will destroy it right away.
The COVID-19 vaccine specifically consists of either spike proteins from the surface of the virus, or Messenger RNA (mRNA) that take effect by stimulating the cells of the immune system to mount a protective immune response specifically against those COVID-19 viral proteins. The antibodies that are produced remain in the bloodstream for years and will immediately act to neutralize the COVID-19 virus if the person gets infected. This way, anyone who receives the COVID-19 vaccine is automatically protected from getting sick with the disease, even if the person gets infected by the virus.
It is important to know that vaccines are a great way to stay protected from getting infected by infectious pathogens. The use of vaccines have helped to greatly reduce the number of cases and mortality rates of many diseases. Diseases like Cholera, Smallpox, Polio and Ebola have been eradicated completely or to very minimal numbers, thanks to worldwide vaccination campaigns against these diseases. The lesser the number of infectious diseases we have to treat, the lesser the strain on our healthcare system. When people get vaccinated against COVID-19, it protects them from getting the infection and falling sick, and also serves to protect other people around by achieving herd immunity. Herd immunity is a phenomenon where a large percentage of the people in a community are immunized against a particular disease, which reduces the likelihood of disease transmission and automatically protects the few individuals that are not immunized. For herd immunity to be attained, about 80% of the community population has to be vaccinated, which is the reason why community and nationwide vaccination is important.
Before any vaccine is approved for use, it must undergo a number of clinical trials (usually three stages) to confirm its safety and efficacy. From clinical trials conducted by the vaccine producers Pfizer and Moderna, it was affirmed that the COVID-19 vaccine is 95% effective against the virus. Concerning the safety of the vaccine for different age groups, there are more concerns about vaccine administration among children. So far, the Pfizer vaccine is the only vaccine approved by the U.S. Food and Drug Administration (FDA) for use among children between the ages of 12-17, which must be administered with full consent from the parent or guardian of the child.
Despite the high level of effectiveness of the COVID-19 vaccine, there have been some side effects observed in a very small percentage of the population. The observed side effects are a sore arm (mainly at the site of vaccine administration), and sometimes slight flu-like symptoms like fever, chills, headaches and tiredness for a day or two. These side effects are rare, they are usually not severe, and are simply a sign that the body is building an immune response against the virus. The likelihood of severe adverse reactions (anaphylaxis) is very low, estimated at about one person in every 90,000 people vaccinated. Regular surveillance is being carried out to monitor any side effects or reactions post-vaccination, and strategies are being implemented by the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) to follow up with such cases of vaccine reaction.
World Health Organization (2021). COVID-19 Advice for the Public: Getting Vaccinated. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice
Johns Hopkins Medicine (2021). Full FDA Approval of a COVID-19 Vaccine: What You Should Know. https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/full-fda-approval-of-a-covid-19-vaccine-what-you-should-know?amp=true
Centers for Disease Control and Prevention (2021). Types of COVID-19 Vaccines Available. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html
UC Davis Health (2021). How the COVID-19 Vaccine Works, Potential Side Effects and More. https://health.ucdavis.edu/coronavirus/covid-19-vaccine/how-covid-19-vaccines-work.html
Website was created with Mobirise